Pipeline
PulseSight’s pipeline comprises clinical- and preclinical-stage programs built on over a decade of development, supported by robust preclinical packages and Phase 1/2 clinical studies confirming the potential of PulseSight’s approach1 in terms of activity and safety.
A Clinically Validated Platform with Multiple Applications
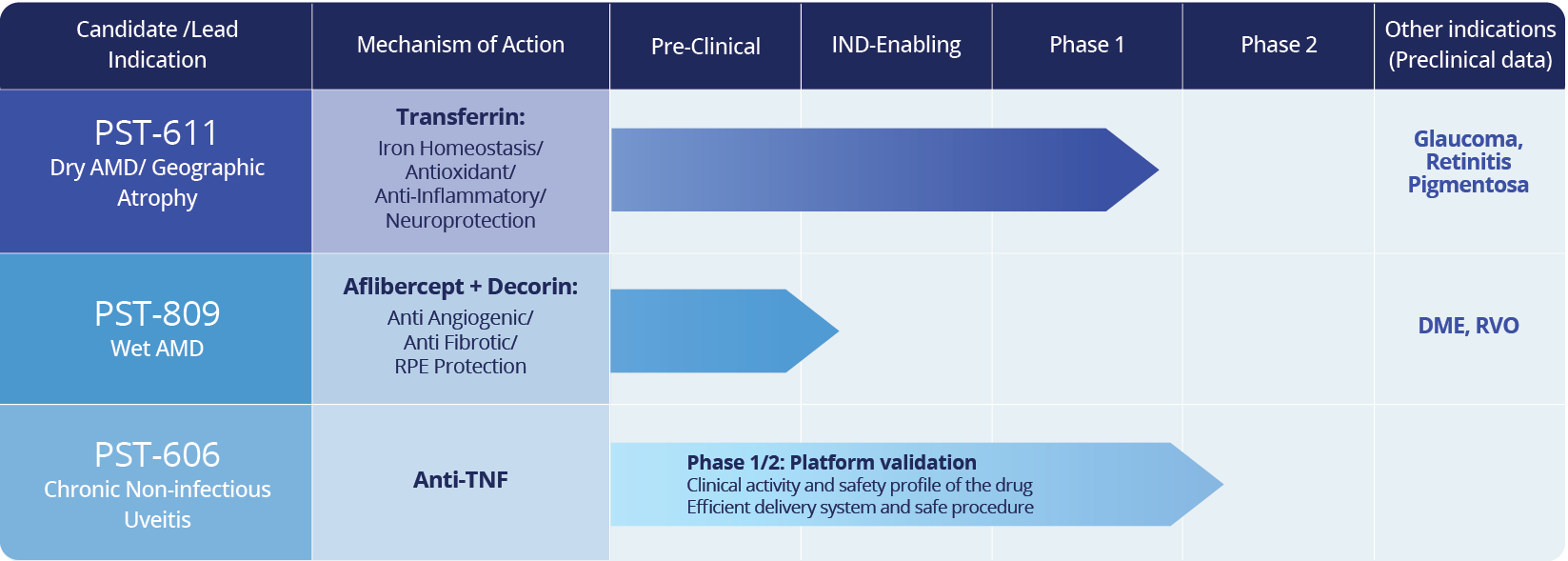
- Hoogewoud et al. EYS606 for the Treatment of Non-Infectious Uveitis. Acta Ophthalmologica 99(3): 343 (2019)