PST-611 – in late-stage dry Age related Macular Degeneration (AMD)/Geographic Atrophy (GA).
Age-related Macular Degeneration (AMD) is the leading cause of central vision loss in individuals over 50 years old; geographic atrophy (GA) is the advanced form of dry AMD and leads to irreversible blindness.
GA is characterized by localized photoreceptors and retinal pigment epithelium (RPE) cell loss. and choriocapillaris atrophy. GA affects about 5 million people globally and ~1.5 million in europe,1 and still represents a high unmet medical need.
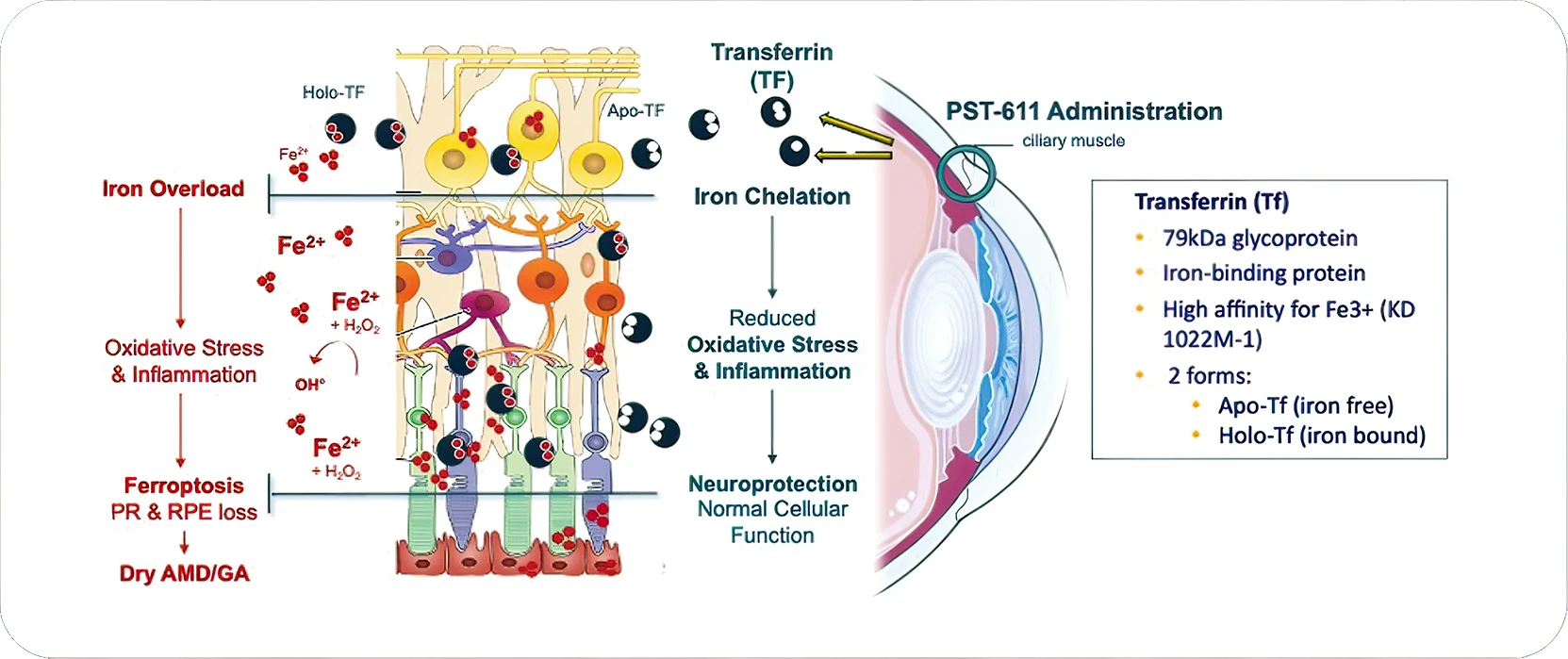
PST-611 encodes human transferrin, a natural iron-binding protein with a key role in controlling normal iron levels in the eye.
Iron is needed for retinal cell metabolism and visual function, but excess of free iron is highly toxic to the retina, causing oxidative stress, lipid peroxidation, mitochondrial damage, and inflammation, leading to ferroptosis and cell death.2
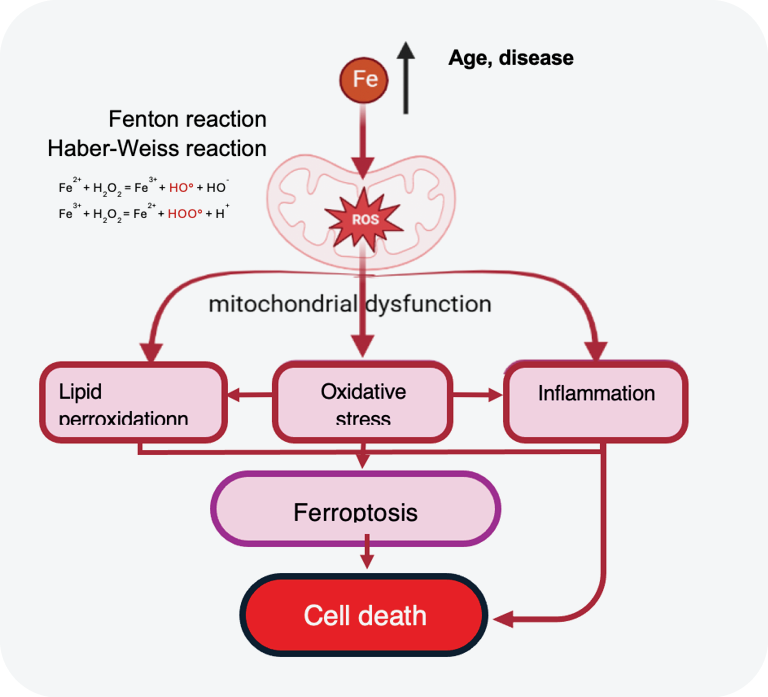
With age and AMD, iron homeostasis dysregulation leads to an excess of free iron causing highly toxic effects, inducing photoreceptor and RPE loss.3
Iron overload is associated with AMD: GA patients show elevated iron levels in the aqueous humor compared with controls4,5 and AMD retinas display increased levels of iron vs. healthy controls.6,7
Acting as a major iron regulator, transferrin plays a key role in the normal iron homeostasis, binding and controlling Iron import to the cells, thus ensuring proper level of free iron / bound iron.
PST-611, coding for human transferrin, restores normal iron homeostasis and prevents the iron-induced toxic cascade.
PST-611 Benefits
- Acting upstream, the transferrin supplementation leads to the inhibition of all downstream iron-related pathological cascade: Oxidative stress; Mitochondrial damage; Inflammation, including complement activation and Ferroptosis.2
- Preclinical data have demonstrated PST-611 ability to protect Photoreceptors and RPE cells in aggressive animal models as well as preserve the retinal function.8
- PST-611’s safety profile is being assessed in a phase I clinical study and results will be presented during the 2026 ARVO Annual meeting in May.
- The sustained expression should considerably reduce the treatment burden, with only 2 or 3 injections/year, improving patient’s compliance.
PST-611 Clinical Status
- PST-611 entered a First-in-Human (FIH) open-label study in June 2025, aiming to demonstrate safety and tolerability profile. Enrolment was completed in late 2025 with final data readout expected in Q2 2026.
- Following the FIH study, PST-611 is planned to progress into a Phase 2a clinical trial shortly after. The phase IIa will assess PST-611 safety and efficacy in GA patients treated up to 3 times over 1 year.
| 1Querques G et al., Ophthalmol Ther 2024 | 5Jünemann, 2013, PLoS ONE 8(2): e56734 |
| 2Ndayisaba, 2019, Front. Neurosci. 13:180; 5 | 6Hahn, 2003, Arch. Ophthalmol. 121:1099 |
| 3Picard, 2020, Cells 9:705 | 7Biesemeier, 2015, Exp Eye Res, 137:39 |
| 4Youale, 2025, Cell Death & Dis. 16:692 | 8Bigot et al. Pharmaceutics (2020) 12:836 |